top of page

The Alkali Metals/Group 1
The Alkali Metals also known as Group 1 are found at the left side of the periodic table(As shown in the picture on the left). The alkali metals are soft and can be cut with a knife, they also contain very reactive metals that can even explode when in contact with water.

The members of the Alkali Metals include:
-Lithium (Li)
-Sodium (Na)
-Potassium (K)
-Rubidium (Rb)
-Caesium (Cs)
-Francium (Fr)
Things the Alkali metals have in common:
-They all have 1 valence electrons, which means that their last outer shell has 1 electron in it. (E.g. lithium's electron shell configuration is:2,1 and Sodium's is 2,8,1 etc.)
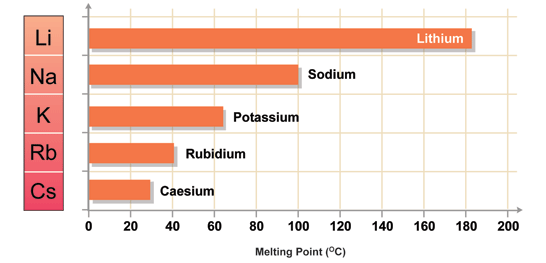
-As you go down the group, their reactivity increases, while their boiling and melting points decrease
Bonding:
-Group 1 metals bond with other elements using ionic bonds which are held together by electrostatic bonds.
-They give their 1 valence electron from their outer shell to the non metal it is going to bond with. -Because it now has more protons(11) than electrons(10), the ion gains a positive charge thus becoming a positive ion.
E.g. the reaction between NaCl:
Reactivity:
All Alkali Metals react very vigorously when in contact with water, in the reactions, Hydrogen gas is given off and hydroxide is also produced, in general:
Metal+Water Metal Hydroxide+Hydrogen
For example:
Lithium+Water Lithium Hydroxide+Hydrogen


bottom of page

