top of page

The Noble Gases/Group 0
Group 0 also known as the Noble Gases are located at the far right of the periodic table and are monatomic(E.g. Helium=He not He )They are inert-Chemically inactive, this means that often times they do not react with other elements. This is because that they have full outer shells which makes them stable.
Elements in Group 0 do not usually form ionic bonds with other elements that is because it already has 8(with the exception of Helium which has 2) valence electrons.Atoms only form bonds just so that it can be stable/Having a full outer shell(Which the gases in Group 0 already have)
Uses:
Helium:Used in balloons
Neon:Used in advertising signs
Argon:Used in light bulbs
Krypton:Used lasers
The members of the Noble Gases include:
-Helium (He)
-Neon (Ne)
-Argon (Ar)
-Krypton (Kr)
-Xenon (Xe)
-Radon (Rn)
2
Noble Gases Trends:
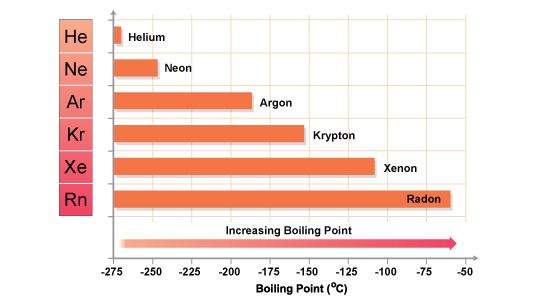
-They all have very low boiling points which is a common property of non-metals
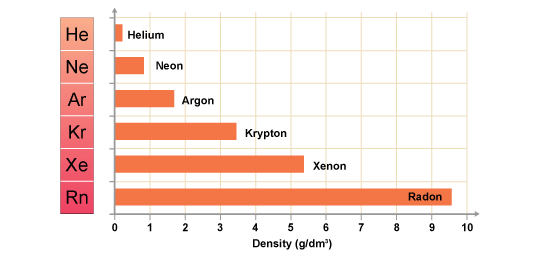
-As you go down the group, the density, boiling and melting points increase
bottom of page

